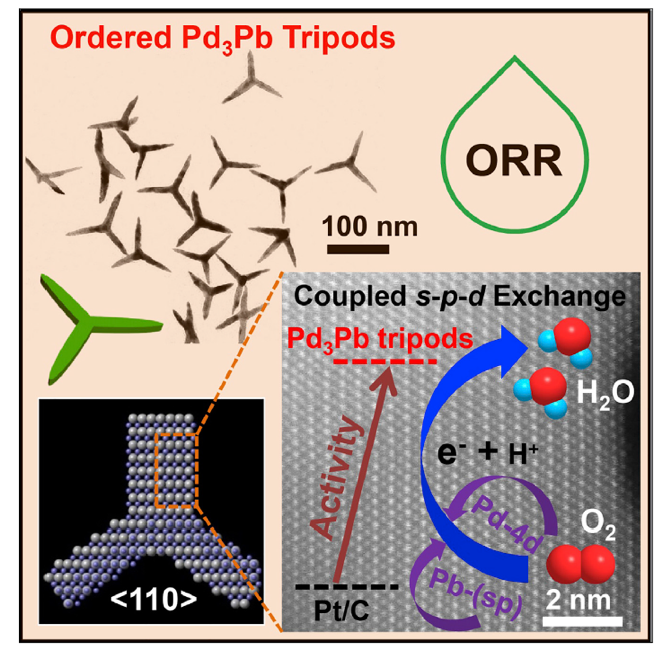
Efficient oxygen reduction reaction (ORR) catalysts are key for the development of high-performance fuel cells. Palladium (Pd) is a promising catalyst system for ORR given its potential to replace platinum (Pt); however, it usually exhibits lower activity than Pt. Here, we report a class of ordered Pd3Pb tripods (TPs) with predominantly {110} facets and show that they achieve extremely high ORR performance in alkaline medium.
In contrast to the knowledge that the excellent ORR activity of Pt catalyst is caused by its partially filled d orbital, our first-principle calculations suggest that the strong charge exchange between Pd-4d and Pb-(sp) orbitals on the Pd3Pb TPs {110} facet results in a Pd-Pb local bonding unit with an orbital configuration similar to that of Pt. Consequently, Pd3Pb TPs exhibit much higher ORR activities than commercial Pt/C and commercial Pd/C. Pd3Pb TPs are endurable and sustain over 20,000 potential cycles with negligible structural and compositional changes.