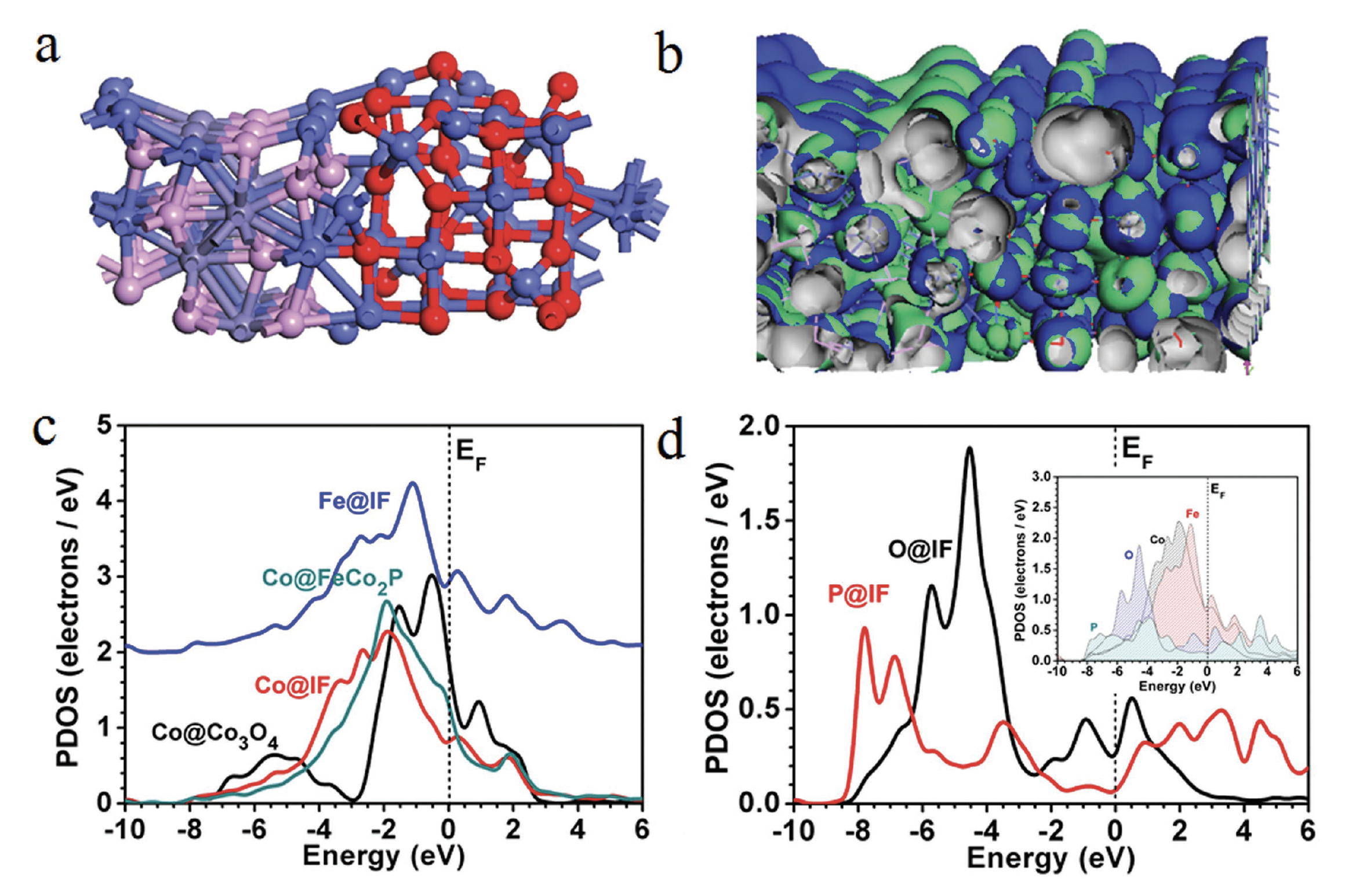
Designing well-defined nanointerfaces is of prime importance to enhance the activity of nanoelectrocatalysts for different catalytic reactions. However, studies on non-noble-metal-interface electrocatalysts with extremely high activity and superior stability at high current density still remains a great challenge.
Herein, a class of Co3O4/Fe0.33Co0.66P interface nanowires is rationally designed for boosting oxygen evolution reaction (OER) catalysis at high current density by partial chemical etching of Co(CO3)0.5(OH)·0.11H2O (Co-CHH) nanowires with Fe(CN)6 3−, followed by low-temperature phosphorization treatment. The resulting Co3O4/Fe0.33Co0.66P interface nanowires exhibit very high OER catalytic performance with an overpotential of only 215 mV at a current density of 50 mA cm−2 and a Tafel slope of 59.8 mV dec−1 in 1.0 m KOH. In particular, Co3O4/Fe0.33Co0.66P exhibits an obvious advantage in enhancing oxygen evolution at high current density by showing an overpotential of merely 291 mV at 800 mA cm−2, much lower than that of RuO2 (446 mV). Co3O4/Fe0.33Co0.66P is remarkably stable for the OER with negligible current loss under overpotentials of 200 and 240 mV for 150 h. Theoretical calculations reveal that Co3O4/Fe0.33Co0.66P is more favorable for the OER since the electrochemical catalytic oxygen evolution barrier is optimally lowered by the active Co- and O-sites from the Co3O4/Fe0.33Co0.66P interface.